Infrastructure
Scotland operates a centralised system to support clinical research and improve quality, efficiency and coordination. Working on a pan-Scotland basis a responsive infrastructure has been implemented to encourage researchers to bring studies to Scotland and ensure that obtaining R&D permission is a smooth and rapid process.
Investments in nationwide clinical research infrastructure also supports researchers, clinicians and industry, providing states of the art facilities to conduct clinical trials.
Management of NHS Research Scotland
Oversight and strategic direction of NHS Research Scotland is achieved through the NRS Strategy Board involving the Chief Scientist Office of Scottish Government and Research and Development Directors from the four lead research Health Boards in Scotland (NHS Grampian, NHS Greater Glasgow & Clyde, NHS Lothian and NHS Tayside).
The Central Management team was established in late 2014 and provides the central point for coordinating and supporting clinical research activity across Scotland. It has five distinct functions – Finance and Data, Academic Liaison, Industry Liaison, Communications and Network Support.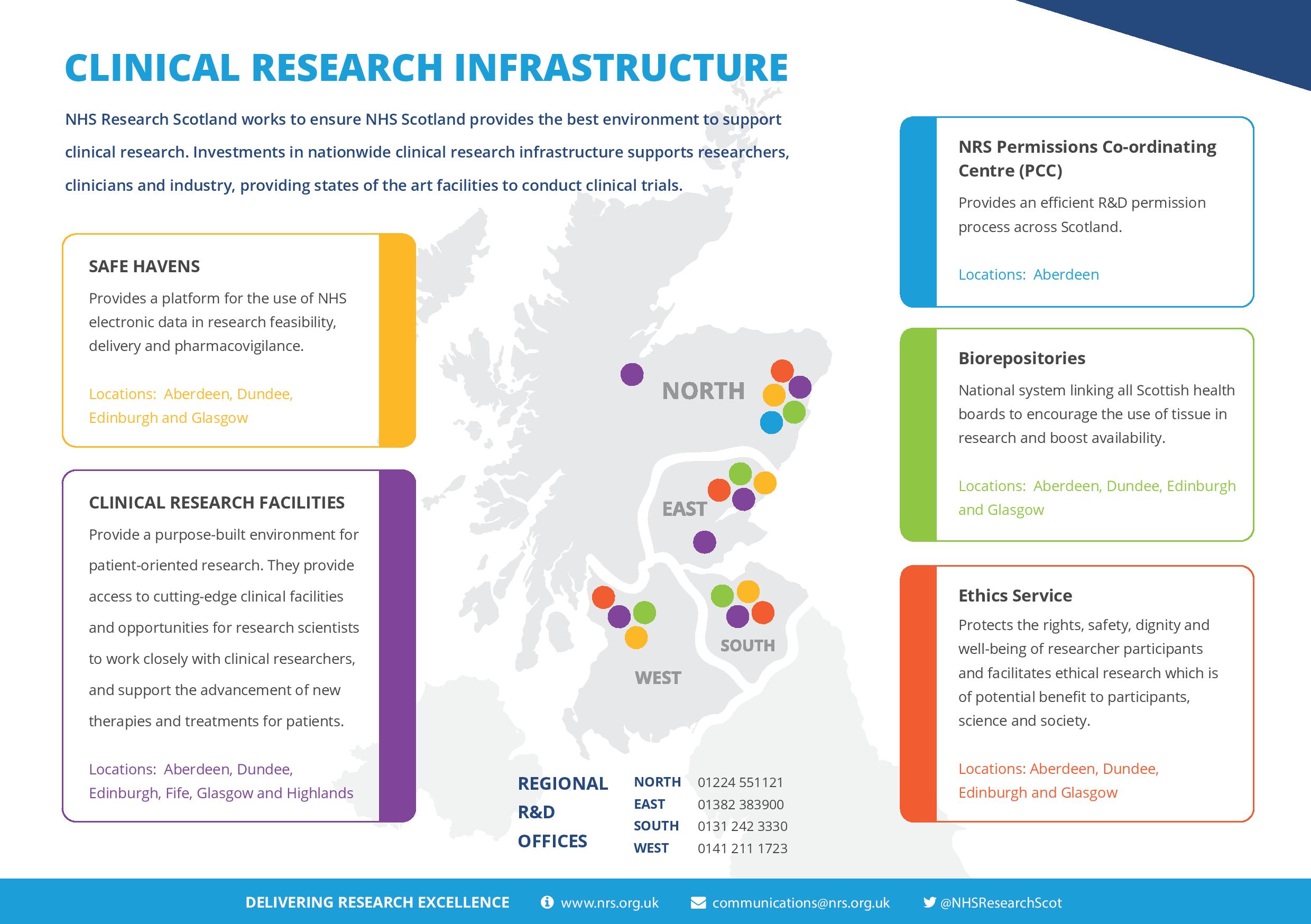
Regional Nodes
Local delivery, development and governance of clinical research are achieved through regional nodes working within NHS Boards to provide support across Scotland.
- North includes NHS Grampian and NHS Highland and is managed from the R&D Office in NHS Grampian. The North node takes the lead on an efficient permissions system by hosting the NRS Permissions Coordinating Centre
- East includes NHS Tayside, NHS Forth Valley and NHS Fife and is managed from the TASC Office in NHS Tayside. The East node takes the lead on Governance issues
- South this includes NHS Lothian and NHS Borders and is managed from the ACORD office in NHS Lothian. The South-East node has responsibility for NRS contractual issues and training
- West this includes NHS Greater Glasgow and Clyde, NHS National Waiting Times, NHS Lanarkshire, NHS Ayrshire & Arran and NHS Dumfries and Galloway. It is managed by the R&D office in NHS Greater Glasgow and Clyde. The West node leads on the IT infrastructure for NRS by hosting the Scottish Research management Database (SReDa) and the National Databases Coordinator.
NRS Network
Delivery of a range of high-quality studies across a spectrum of diseases is provided through the NRS Clinical Research Network and Specialty Groups. The Network and Specialty Groups support the development, set up and completion of clinical research studies across Scotland by providing advice on study design and feasibility, identifying potential sites, facilitating access to resources and monitoring recruitment in specialist disease areas.
NRS Permissions Co-ordinating Centre (PCC)
An efficient R&D permission process across Scotland, for commercial and non-commercial multicentre research projects, is delivered by the PCC. This provides a single point of contact for industry and investigators, centralised project coordination and faster approvals.
Clinical Research Facilities
Located in Aberdeen, Dundee, Edinburgh, Fife, Glasgow and Highland, Clinical Research Facilities provide support in the provision of trained research staff, dedicated clinical research space and expertise in the conduct of clinical trials.
Biorepositories
Each NRS node hosts a Biorepository that holds responsibility for tissue governance for their partner board and supports and facilitates access to surplus diagnostic and surgical tissue for use in research. These nodes form a network of Biorepositories designed to encourage the use of tissue in research and boost the availability of tissue from across Scotland.
Data Safe Havens
Available in each NRS node, Safe Havens provide a 'secure' platform for the use of NHS electronic data in research feasibility, delivery and pharmacovigilance.

